X-ray fluorescence analysis, XRF: The CRB-Analysis

The CRB-Analysis: Preparation of a fused bead for XRF by means of a gas burner
We offer all standard preparation procedures and analytical techniques for quantitative and semi-quantitative XRF-analysis of a wide variety of materials for up to 71 elements.
The CRB-Analysis: Our services in the field of XRF
- Different preparation procedures (fusion, powder pellet, non-destructive measurements)
- Fast and reliable analyses with high precision and accuracy down to the trace elements, if requested on-the-spot
- Broad range of elements up to 71 elements from fluorine to uranium in one single measurement run, suitable for the monitoring of products and raw materials. Analyses of unknown materials.
- Accredited testing procedures
Important links:
Further information
X-ray fluorescence analysis, XRF – basics
 XRF is employed for the qualitative and quantitative analysis of liquids and solids in order to determine their chemical composition. It is frequently used in the metal industry for examining glass, ceramics, building materials as well as for analysing lubricants and mineral oil products. Practical detection limits are around a few mg/kg.
XRF is employed for the qualitative and quantitative analysis of liquids and solids in order to determine their chemical composition. It is frequently used in the metal industry for examining glass, ceramics, building materials as well as for analysing lubricants and mineral oil products. Practical detection limits are around a few mg/kg.
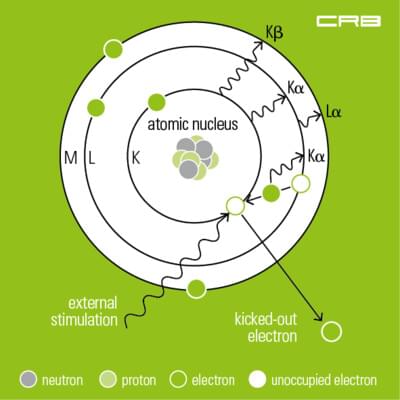
In X-ray analysis the material to be analysed is excited by a primary source, i.e. by polychromatic X-rays, gamma or ion radiation generated in the X-ray tube. Excitation by an electron beam/cathode ray is used in X-ray microanalysis, EDX.
During the measurement inner-shell electrons located near the nucleus are raised to the outer shell. As a result electrons from higher energy levels fill the vacancies. The excess energy which was created during this process is given up as an X-ray. Thus every element has its characteristic fluorescence radiation consisting of one or more fluorescence lines with a specific energy level – compare Mosley’s Law.
Depending on its construction and the way fluorescence radiation is detected there are energy-dispersive and wavelength dispersive spectrometers.
Analysis by wavelength dispersive X-ray fluorescence spectrometer, WDXRF
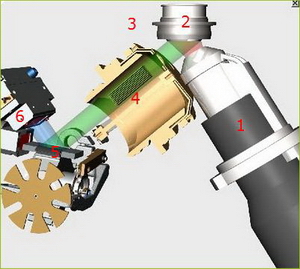
Functional principle
In case of wavelength dispersive X-ray fluorescence, WDXRF, the excitation is achieved by primary radiation of an X-ray tube. The radiation emitted is collimated by parallel copper blades, diffracted by a crystal and collected in the detector.
The crystal serves to diffract the specimen’s spectrum of polychromatic secondary radiation. It allows the qualitative determination of single elements according to the diffraction angle and the quantitative determination by measuring the intensity of the secondary radiation.
Principle of operation of a WDXRF
X-ray source (1)
Usually an X-ray tube is used as X-ray source:
- side-window type: An anode made of chrome, tungsten, molybdenum, gold or rhodium is bombarded with an electron beam. Very much heat is generated as well as X-rays which leave the X-ray tube through the beryllium window.
- An end-window type is more common due to its higher radiation density. In this case the anode is located opposite the beryllium window, the cathode is build up around the anode in a ring. When the X-ray tube is energised by a high-voltage power supply the electrons migrate towards the anode.
Specimen (2)
Tubefilter (3)
The X-ray spectrum produced by electron bombardment consists of the “bremsstrahlung”, i.e. the deceleration of electrons by the atoms in the target and the characteristic line spectrum of the material used for the anode in the X-ray tube. In order to absorb the lines of the anode a primary filter is used with an atomic number of 1 or 2 less than the anode material, e.g. a titanium filter for a Cr-Tube.
Collimator (4)
A stack of parallel metal plates (Soller plates) used for scattering, i.e. for selecting a parallel beam of rays from a diverging fluorescence radiation.
The analyzing crystal (5)
When passing the analysing crystal the polychromatic X-rays are bent in such a way, that at a certain angle of incidence/angle of reflection only radiation of a certain energy or wavelength is reflected. The basis of this principle is Bragg's law.
Detection of the fluorescence radiation (6)
The detection of fluorescence radiation is done by scintillation for heavy elements with highly energetic, short-wave characteristic radiation, and by gas flow proportional detectors for light elements with a less energetic, long-wave-characteristic radiation.
After correcting matrix effects and line overlaps, the intensity of the characteristic radiation of each element shows its concentration in the specimen.
Energy dispersive X-ray fluorescence spectrometer, EDXRF
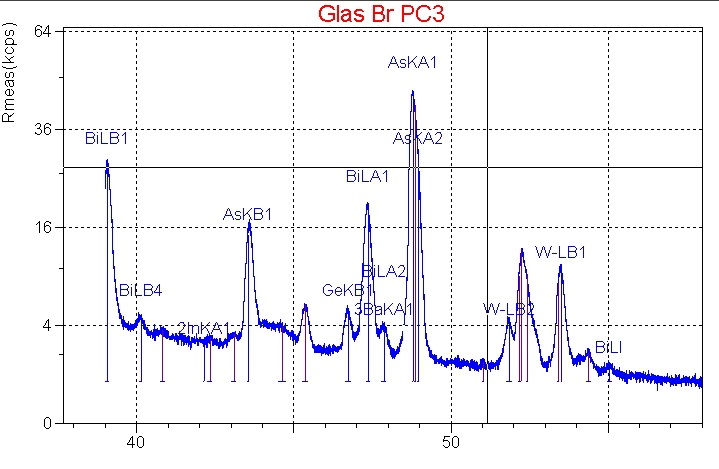
As in case of WDXRF, the excitation of the elements in the sample is also achieved by primary radiation of an X-ray tube – compare XRF analysis – basics.
The characteristic fluorescence radiation emitted by each element in the sample is registered by a detector, usually a semiconductor crystal made from silicium or lithium, a SiLi-detector, or a silicium-drifted detector, SSD.
By means of suitable electronic devices the signal emitted by the detector is converted in such a way that it can be analysed by a Multichannel Analyzer, MCA.
It records the incoming pulses, the protons that were previously detected in relation to their amplitude, i.e. the amount of energy they are emitting.
The result is an energy-dispersive element spectrum.
Using suitable evaluation software, the elements of the sample and their concentrations can be determined on basis of the data obtained during the detection process.
X-ray microanalysis, EDX
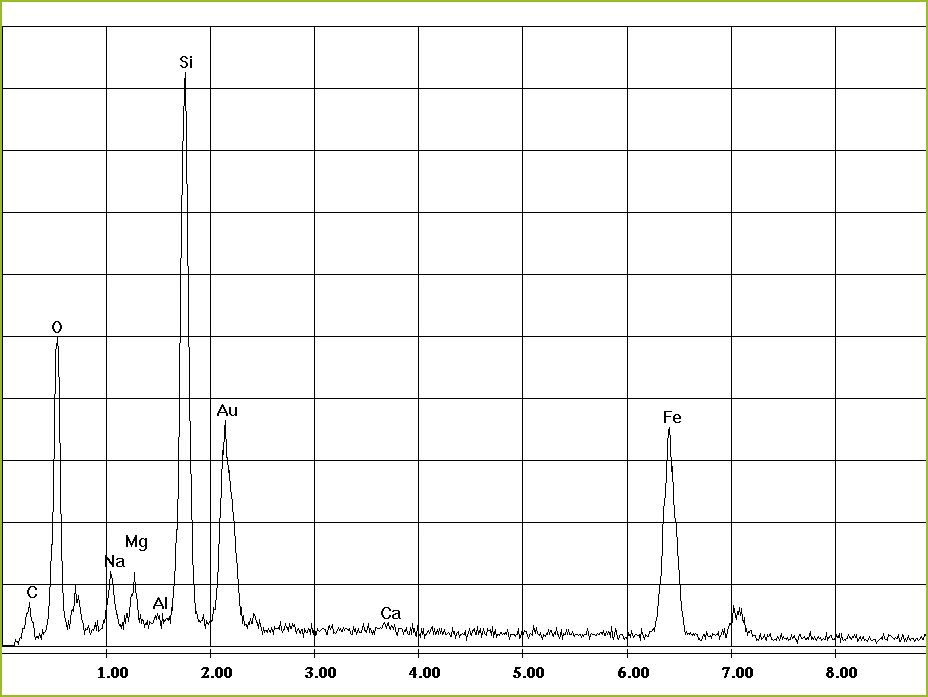
Its functional principle is similar to that used in energy-dispersive XRF only in this case the high-energy primary electron beam is used to excite the elements in the sample.
The characteristic fluorescence radiation emitted by each element in the sample is registered by a detector, usually a semiconductor crystal made from silicium or lithium, a SiLi-detector, or a silicium-drifted detector, SSD.
By means of suitable electronic devices the signal emitted by the detector is converted in such a way that it can be analysed by a Multichannel Analyzer, MCA.
It records the incoming pulses, the protons that were previously detected in relation to their amplitude, i.e. the amount of energy they are emitting.
The result is an energy-dispersive element spectrum.
- Qualitative analysis via EDX spectra
There are several lines for most elements in the spectrum. With regard to the classification of lines it must be checked if all lines of a specific element are present and if their intensities are inversely proportional. At the same time peak overlaps with other elements must be considered. The energy resolution of an EDX spectrometer imposes limits on the separation of neighbouring peaks. Kα1 and Kβ2 lines of an element are commonly affected but also lines of different elements. - Quantitative analysis
The quantitative determination of the intensity of characteristic X-ray lines is done by integrating the lines and subtracting the continuous background produced by the bremsstrahlung. Initially only raw values for the mass percentages are resulting from the relative intensities of the X-ray lines of different elements, because the number of the registered characteristic X-ray quanta are depending not only on the concentration of the element but on a number of further material parameters, that are accounted for by means of ZAF corrections, a procedure in which corrections for atomic number effects (Z), absorption (A) and fluorescence (F) are calculated separately from suitable physical models.
List of standards and guidelines for XRF-analysis
- ISO 29581-2:2010-03 - Cement - Test methods - Part 2: Chemical analysis by X-ray fluorescence
- DIN EN ISO 12677:2013-02 - Chemical analysis of refractory products by X-ray fluorescence (XRF) - Fused cast-bead method
- DIN EN ISO 21068-1:2008-12 - Chemical analysis of silicon-carbide-containing raw materials and refractory products - Part 1: General information and sample preparation
- DIN EN ISO 21068-2:2008-12 - Chemical analysis of silicon-carbide-containing raw materials and refractory products - Part 2: Determination of loss on ignition, total carbon, free carbon and silicon carbide, total and free silica and total and free silicon
- DIN EN ISO 26845:2008-06 - Chemical analysis of refractories - General requirements for wet chemical analysis, atomic absorption spectrometry (AAS) and inductively coupled plasma atomic emission spectrometry (ICP-AES) methods
- DIN EN 196-2:2013-10 - Method of testing cement - Part 2: Chemical analysis of cement
- DIN EN 15309:2007-08 - Characterization of waste and soil - Determination of elemental composition by X-ray fluorescence
- DIN EN 62321-3-1:2014-10 - Determination of certain substances in electrotechnical products - Part 3-1: Screening - Lead, mercury, cadmium, total chromium and total bromine by X-ray fluorescence spectrometry
- DIN 51001:2003-08 - Testing of oxidic raw materials and basic materials - General bases of work for X-ray fluorescence method (XRF)
- DIN 51001 Beiblatt 1:2010-05 - Testing of oxidic raw materials and basic materials - General bases of work for X-Ray fluorescence method (XRF) - General survey on disintegration methods referred to groups of materials for the determination of test specimens for XRF
- DIN 51081:2002-12 - Testing of oxidic raw materials and materials - Determination of change in mass on ignition
- DIN 51418-1:2008-08 - X-ray spectrometry - X-ray emission- and X-ray fluorescence analysis (XRF) - Part 1: Definitions and basic principles
- DIN 51418-2:2015-03 - X-ray spectrometry - X-ray emission and X-ray fluorescence analysis (XRF) - Part 2: Definitions and basic principles for measurements, calibration and evaluation of results
- DIN 51719:1997-07 - Testing of solid fuels - Solid mineral fuels - Determination of ash content
- DIN 51729-10:2011-04 - Testing of solid fuels - Determination of chemical composition of fuel ash - Part 10: X-Ray Fluorescence Analysis










